Mendeleev and the periodic table

At the turn of the nineteenth century, John Dalton proposed the atomic theory. He proposed each element comprises atoms of a single, unique type, and that these atoms could combine in fixed ratios to form compounds. Scientists raced to find more of these “pure” elements - hydrogen, oxygen, nitrogen, and even some metals like gold, silver, and copper had already been discovered - but how many more elements were out there? Chemists all over the world went crazy trying to find additional elements and perhaps gain instant fame from the treasures the new element might bring.
Dmitri Mendeleev was the guy who gave them the treasure map. Dmitri Mendeleev created the treasure map by taking all the different atoms that had been discovered up until that time and placing them in a table using increasing atomic numbers, i.e. the number of protons in an atom. Of course, not all elements were discovered and there turned out to be gaps in the table. Mendeleev was so confident of his table that he said additional elements would be discovered that would fill up the gaps. And that’s exactly what happened. By the end of the nineteenth century, scientists had discovered almost 80% of the elements, and they filled up the “gaps.”
But this is not a story of the periodic table, per se. This is a story of how and when the universe created these elements. So let’s go back in time, almost 13.7 billion years ago, when presumably there is nothing.
And it explodes.
A fraction of a millionth of a second later, quarks, electrons, photons, and other exceedingly small particles come to exist. 10 to the power of -18 meters, or 0.1 zeptometers small. These tiny particles (quite an understatement!) are zipping around at tremendous speeds. This is the quark epoch.
A micro-second later, quarks combine with each other to create protons and neutrons. The hot primordial soup now contains photons, electrons, protons, neutrons, and they keep hitting each other with great forces. This is the Hadron epoch. An observer at a distance would see just darkness, because the photons that allow us to see the universe would just keep getting ‘hit’ by the electrons and bounced around.
The periodic table is still empty.
One minute after the Big Bang, protons and neutrons combine to form the first nuclei. The Universe now has “matter” - mostly hydrogen and helium ions, with trace amounts of lithium ions. There is no solid or liquid matter at this point. Just gas. Positively charged nuclei floating all around.
This is the Big Bang Nucleosynthesis epoch. Atoms have not formed yet because atoms require electrons…and the electrons are moving too fast for the nuclei to catch and hold them down. But why are the electrons moving so fast?
Because it is still too darn hot. A minute has passed by and the periodic table of elements is still…empty.
About 380,000 years after the Big Bang, the universe cools down, so much so that the once fast-moving electrons are now sluggish. The positively charged and heavier protons catch them, creating the first "neutral" atoms. With fewer free electrons to scatter photons, the Universe becomes transparent to light for the first time. An opaque universe has turned transparent. If you were around, you would see a uniform glow from all directions, in a color similar to that of a dim red light.
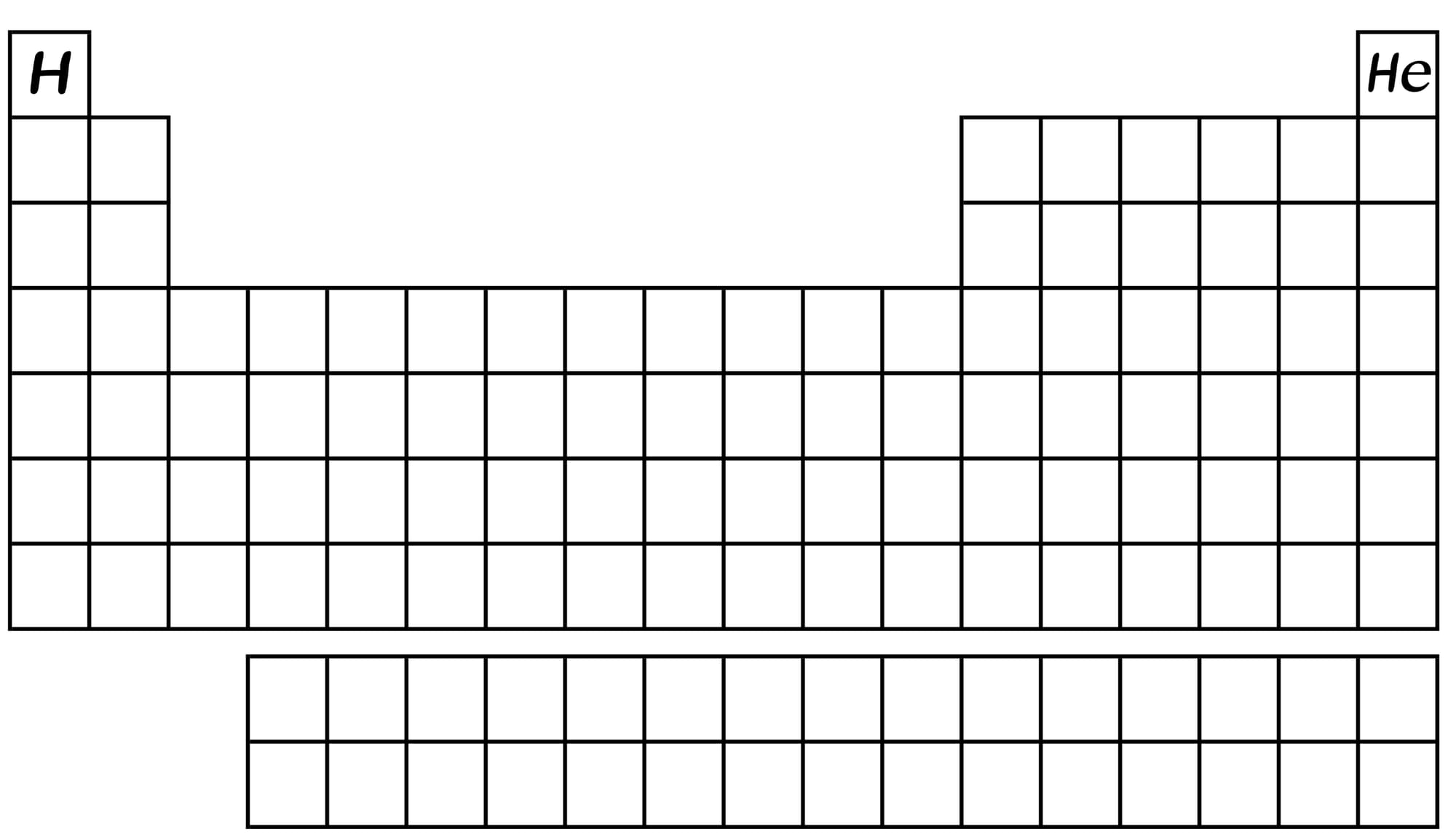
Now, atoms have mass, and anything with mass is pulled by gravity. For some strange reason, luckily for us, the atoms are not evenly spread out. So regions with more atoms begin "clumping" together, creating the first stars. Between these stars, "empty" space forms, devoid of matter.
Deep inside the first baby stars that are now about 100 to 200 million years old, crushing pressure (imagine being in a metro car at MG Road, Bangalore, on December 25th... yes, like that) fuses helium atoms to create heavier elements…and soon we get the next set of elements.
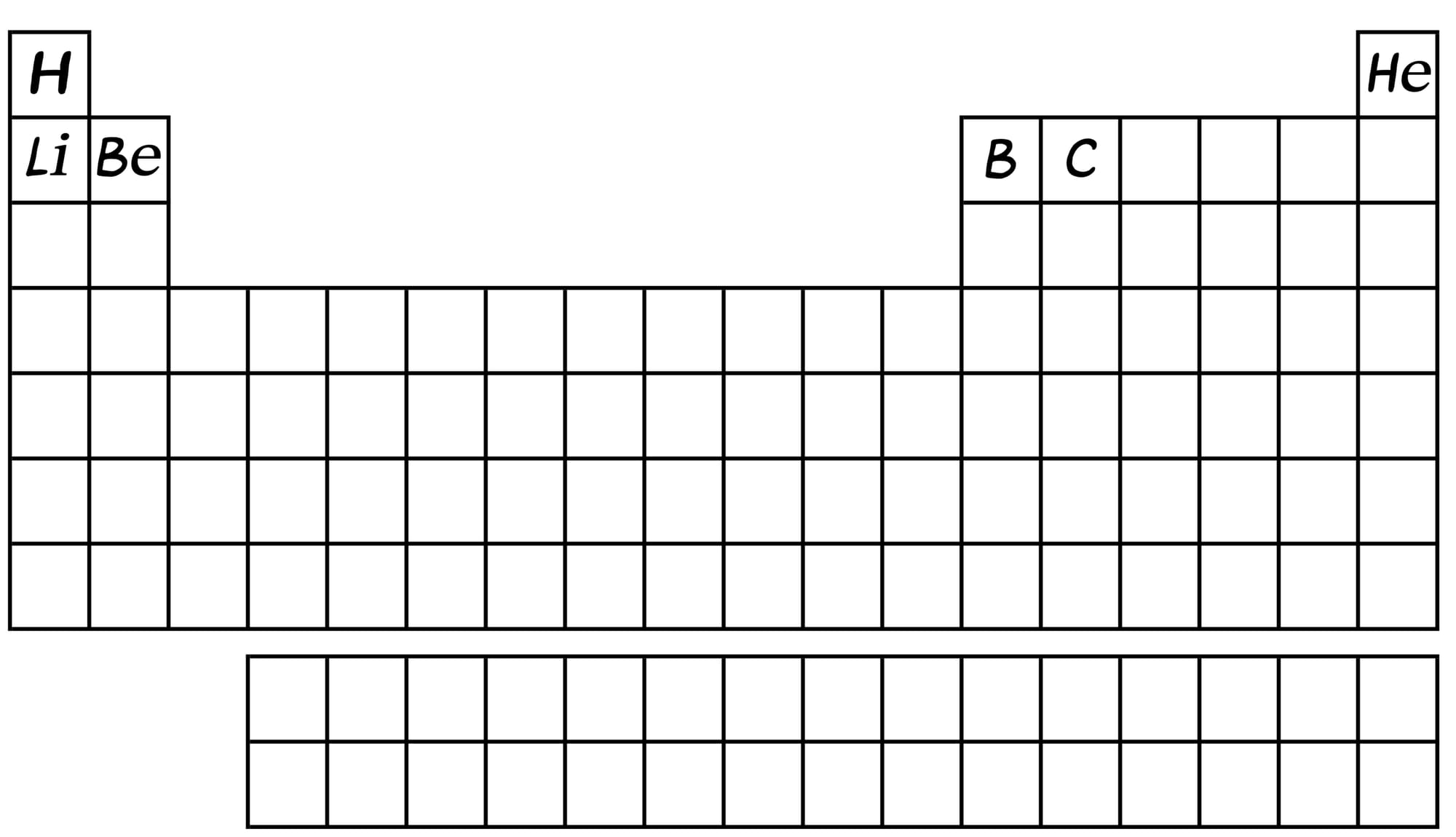
A star the size of our Sun can generate all the elements in the periodic table up to carbon in this manner. With time, and the ever-present force of gravity, massive stars form, such as Betelgeuse. Such stars have so much pressure in their core that they can create even more elements - all the way up to iron.
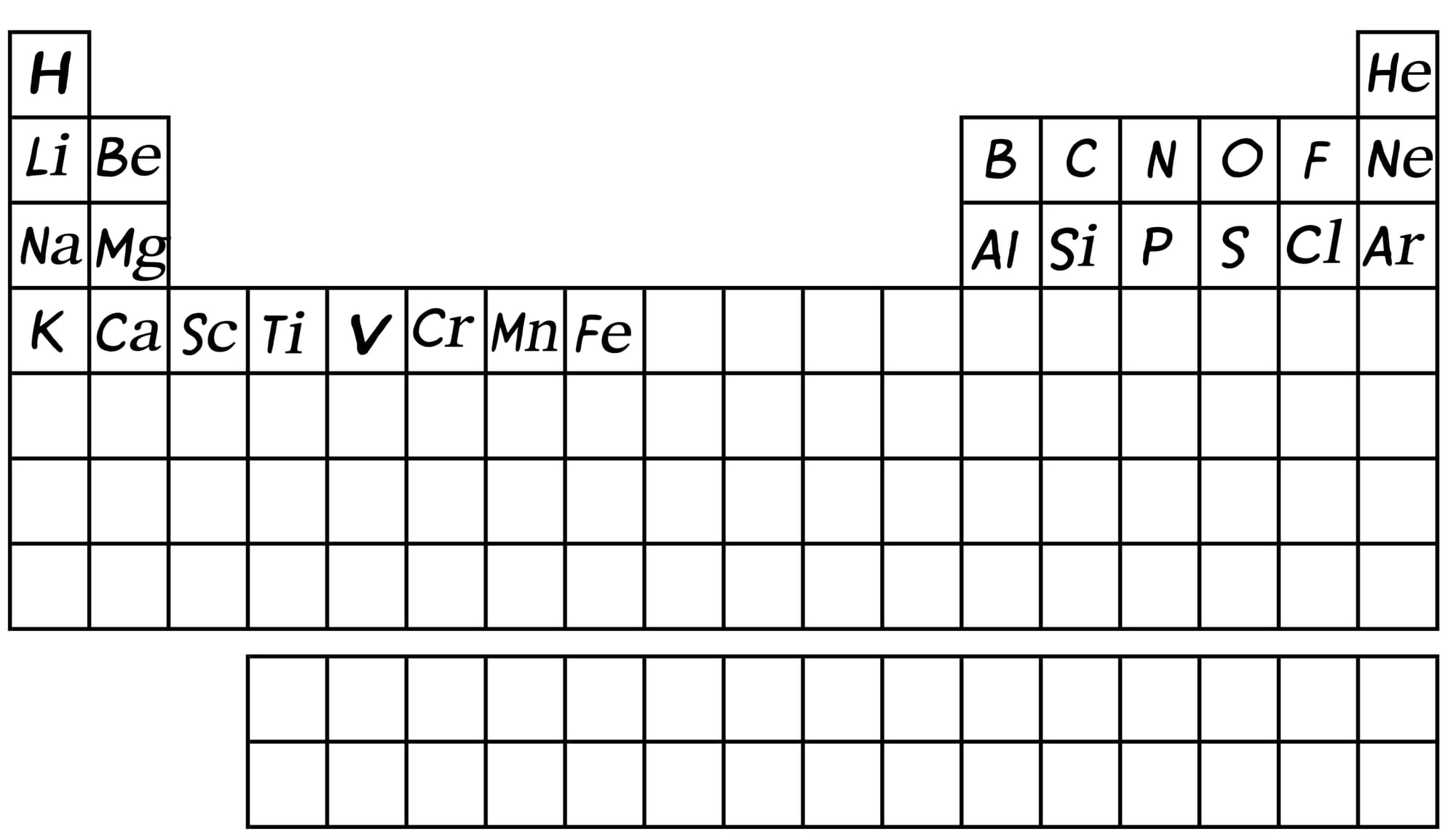
Iron, with an atomic number of 26, means it has 26 protons and almost an equal number of neutrons, all packed into the nucleus. This is the maximum that even the largest stars in the Universe can pack into one nucleus. So where do the rest of the elements in the periodic table come from?
A time comes when these massive stars can grow no more, and they explode in an event called a “Supernova”. The heat and pressure created in a Supernova is so intense that it creates the rest of the elements in the periodic table - all the way to Uranium, which has 92 protons and over 140 neutrons, stuck together in the nucleus.
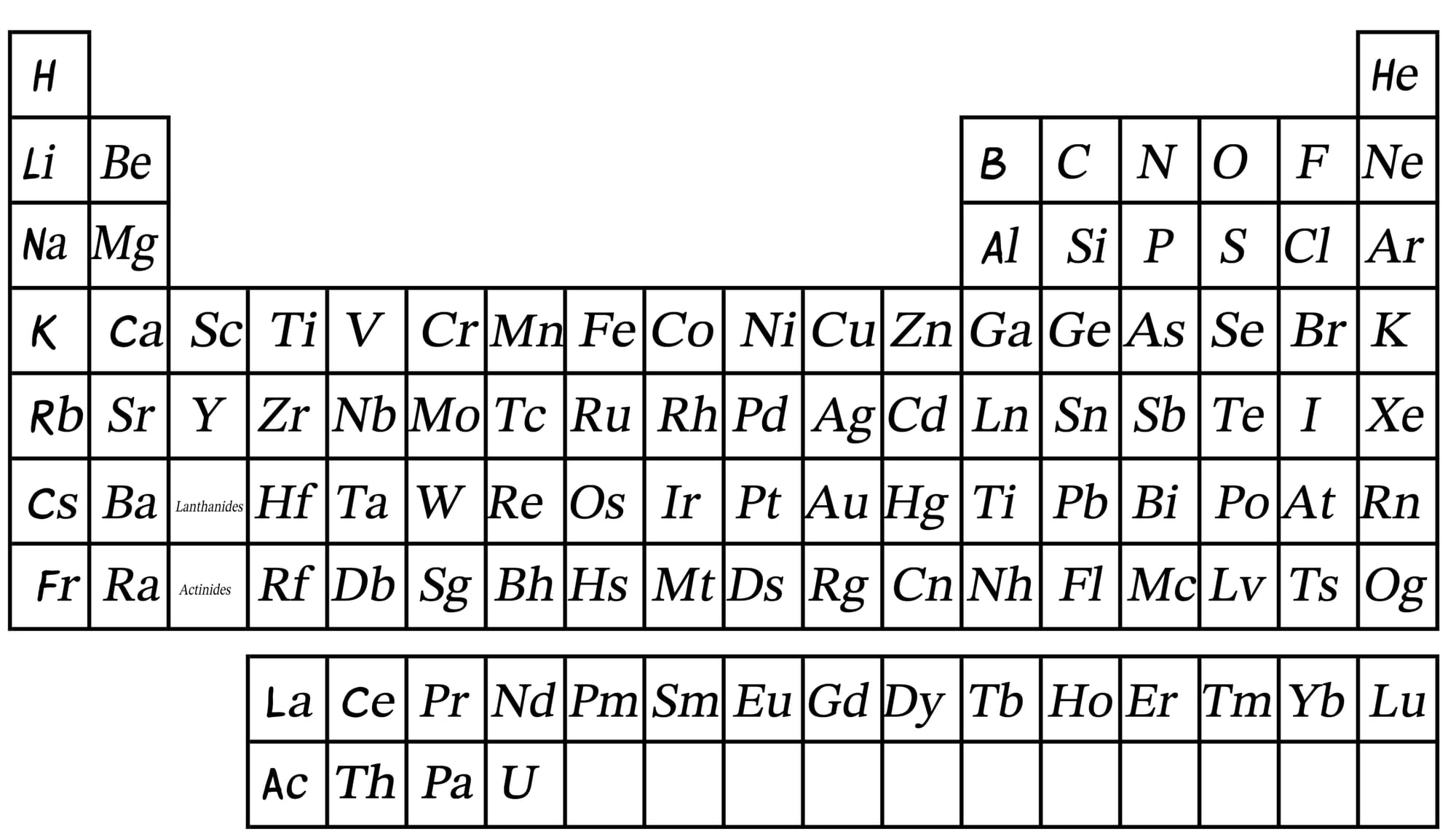
Of course, the star has exploded, so there is nowhere for these elements to go except being scattered all around space. Then, if a new star is being formed in that region, these elements become part of the new star.
So, although the heaviest elements exist in all stars, they contain elements that might have come from another star that exploded way back in time.
And that’s how ladies and gentlemen, boys and girls, are how the elements in the periodic table, and in the universe at large, came to be.


